Project scope by Disease and by Modality
Project scope by Disease
Malaria
- Identification and characterization of molecules with novel antimalarial modes of action using both phenotypic and target based approaches - to enable selection and development of safe potential drugs promoting fast clearance of parasites by the host and/or blocking transmission
- Understanding mechanisms of antimalarial resistance and the development of assays to anticipate impact in the clinical setting
- Antimalarial PK/PD translational models to understand key parameters affording a drug its antimalarial effect
- Assess new culture systems for Plasmodium species including P. vivax blood and liver stages -To enable screening and identification of anti-hypnozoite drugs and to ensure 'pan-active' activity of antimalarials in all human Plasmodium species
- Translational in vitro and in vivo models to assess transmission blocking potential
- Evaluation of combination regimens through development of in vivo, in vitro and ex vivo models
- Novel antimalarial approaches to enable identification of molecules for chemoprotection and chemoprevention including translational models to estimate impact of interventions
Tuberculosis
- Exploitation of novel small molecule screening approaches beyond commonly employed phenotypic strategies (i.e. more relevant to mycobacterial survival in the host)
- Access to unexplored chemical space, natural products and other sources of compounds as hits/leads in TB discovery programs
- In vivo technologies and disease models with increased translational value
- ATarget based approaches on genetically and chemically validated TB and host targets
- Novel approaches to screen and identify additive or synergistic drug combinations
- New technology/tools to accelerate lead optimization campaigns in target-based/phenotypic projects.
- Approaches evaluating the role of immunomodulation in TB
Antimicrobial resistance
- Focused on Bacterial or Fungal pathogens affecting Low and middle-income countries.
- Exploitation of new targets and mechanisms.
- Exploitation of novel phenotypic in vitro assays that allow identification of hits or new combinations with potential to improve standard of care treatment (single cell cultures , co-cultures, organoids, etc.).
- Medicinal chemistry optimization of hits/leads with potential to deliver drugs to prevent/treat infections affecting Low and middle-income countries.
- Development and validation of animal models that reproduce clinical setting, which allow drug evaluation and establishment of PK/PD relationships.
- Development and validation of biopharmaceutical, microbiome-based and non-traditional protein-based approaches.
- Using novel synthetic biology, data science and artificial intelligence approaches in the design of fit-for-purpose treatment options for addressing AMR challenges.
- Delivery systems that can overcome the hurdles associated with these approaches.
Note: Proposals related to Kinetoplastids-mediated diseases are precluded due to the current lack of facilities in Tres Cantos.
Project Scope by Financing
Projects on scope include innovative approaches in the field of endemic infectious diseases that could offer a new solution to an existing gap that can benefit from collaboration with the pharma industry in the fields of gut health (including bacterial enteric infections and environmental enteric dysfunction, EED), malaria, tuberculosis, and kineto mediated infections.
In addtion to discovery projects, since April 2019 TCOLF is accepting applications in the translational and clinical (up to Ph2a) space.
Two funding schemes are currently operating:
- Discovery: This funding stream is a continuation of the original TCOLF model (discovery projects in which co-location is a key factor to be eligible) aligned with scientific priorities. The allocated budget for a Discovery project is expected to be ≤ £200K (exceptions will always be considered e.g. when price of consumables or assays are extraordinarily high, assuming that there is strong scientific rationale and high potential impact of the project).
- Preclinical & Clinical Development: Due to the greater anticipated cost of these projects, the candidates will be restricted to: advanced lead molecules (robust in vivo activity on relevant models with a clear developable profile), clinical candidates, and repurposing opportunities. The allocated budget for these projects is expected to be ≤ £500K. Unlike a Discovery project, co-location is not expected for this funding stream. However, the support will go beyond the financial provision from TCOLF as GSK will continue providing support (e.g. technical input).
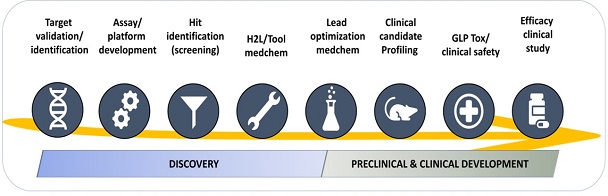
Project scope by Modality
Target validation / identification: Projects on scope include generation of chemical probes for chemical validation of novel genetically validated targets and the use of chemoproteomic approaches for target identification/engagement.
Assay / platform development: Development and miniaturization of quantitative assays for screening (LTS, MTS, HTS) or medicinal chemistry programs.
Hit Identification:Screening campaigns of focus compound sets and / or diversity compound sets from the GSK collection.
H2L / tool optimization: Program focused on optimizing a molecule (in vitro active with none or poor in vivo activity) to deliver a selective (in the case of target base programs) advance analog with a Pharmacokinetic compatible with an oral dosing for proof of concept in animal model (lead compound).
Lead optimization: Medicinal chemistry program focused on optimizing a lead/s molecul/s (in vivo active) to deliver a new analog with a robust in-vivo efficacy subjected to full in vivo pharmacological profiling for the target indication and a tailored toxicological safety assessment to finally qualify as a clinical candidate. Depending on the candidate-likeness of the lead molecule, the project can be considered Discovery Projects or Preclinical & Clinical Development Projects.
Clinical candidate profiling: Tailored plan to evaluate potential clinical candidates (small molecules, biologicals, etc). Projects on scope include non-GMP scale-up, tailored non-GLP in vivo preclinical safety and/or efficacy studies.
GLP Tox / clinical safety: Tailored plan to evaluate clinical safety of novel candidates (small molecules, biologicas, etc). Projects on scope include GMP scale-up, tailored GLP in vitro and in vivo safety studies and Phase 1 studies.
Efficacy clinical studies: Tailored Phase 2 studies.


